Pregnancy and Reproductive Endocrinology
Pregnancy Overview
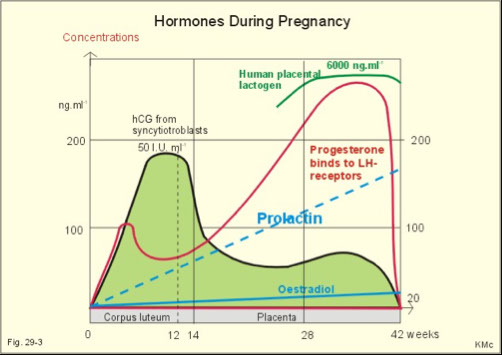
hCG
Estriol
Progesterone
Human Placental Lactogen
Fetal Distress and Monitoring Well-Being
aFP
Bilirubin
Fetal fibronectin
Surfactant
Reproductive Endocrinology
Females - anatomy
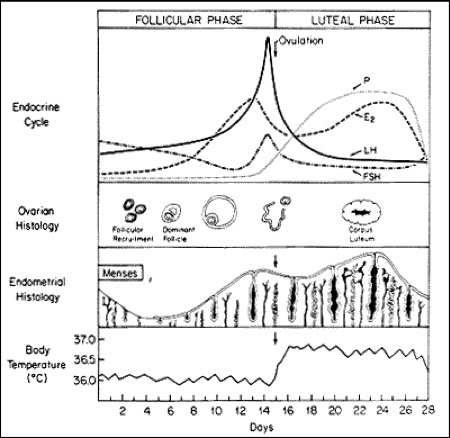
Hormone regulation
Females at puberty
Estradiol
Progesterone
Contraception
Female infertility syndromes
Abnormal ovary function (primary, secondary, and hyperfunctioning)
Investigation of female infertility
- Gonadotropin release testing
- Clomiphine citrate testing
- Progesterone withdrawl test
Male anatomy and hormones
Spermatogenesis
Pregnancy Overview
Trophoblast attaches to the uterine wall approximately 7 days post fertilization.
Maternal & fetal endocrine glands control hormones
Elevated estrogen & progesterone suppress LH & FSH & stimulate flow of prolactin.
hCG
Detectable post implantation
Rapid rise 8-12 weeks
Levels double every 48 hours
10-15% drop maintained till delivery, remains for about 2 weeks postpartum
- inc levels of hCG assoc c inc risk of Down syndrome
- women >35 yo routinely offered invasive prenatal diagnostic testing (usually amniocentesis, which results in fetal loss in 1/200 procedures)
Detect pregnancy in Urine or Serum
6 - 10 days post conception
- can get false negative with dilute urine or a false positive with heterophile antibody interference
Ectopic pregnancy
Extrauterine pregnancy – implantation in fallopian tubes to curnu - 1% of pts c fertility enhancement
Serial measurements of hCG are required
hCG levels do not elevate as high or as fast as true pregnancies - can rupture at any level (even <100)
- can get serum progesterone level (<5 = ectopic)
Hydatiform mole
hCG levels will increase with no apparent pregnancy, but levels are persistently low
- higher in complete than partial moles
Following a D & C serial hCG levels are used to verify the removal of all molar tissue - though can remain at low "phantom" levels for long periods of time
Pregnancy-Associated Plasma Protein A (PAPP-A)
Decreased levels of PAPP-A before the 14th WGA assoc c inc risk of Down syndrome and trisomy 18 (although low levels are also seen in trisomy 13, and other chromosomal anomalies)
- inc levels of PAPP-A (~1.86 Multiple of the Mean (MoM) seen in twin gestation
- inc levels of hCG assoc c inc risk of Down syndrome
- fetal nuchal translucency should also be performed by US as a third maker for fetal anomalies (is increased in majority of cases of Down syndrome)
- PAPP-A is produced by the placental syncytiotrophoblasts and deciduas
- chemically, it is a glycoprotein secreted as an active protease
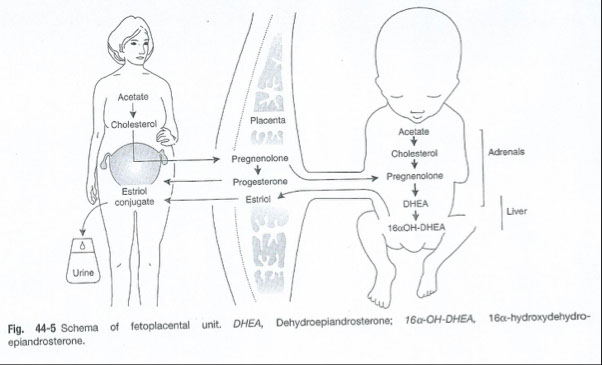
Estriol
Major estrogen in pregnancy
Found in the urine of pregnant women
Made by placenta
Requires the fetal adrenal gland to provide necessary precursors
Levels reflect the growth of the fetus
Stimulates growth of the uterus and mammary glands
Indicates fetal distress when levels drop sharply
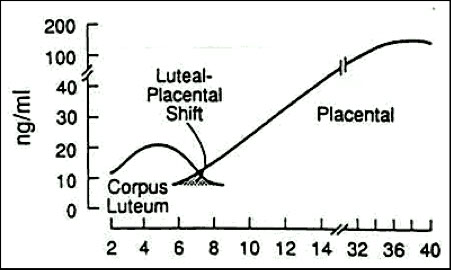
Progesterone
Indicates viability of the placenta but not the fetus
Inhibits uterine contractions
Stimulates mammary gland development
Human Placental Lactogen
Stimulates estrogen & progesterone
Stimulates development of mammary glands
Fetal Distress and Monitoring Well-Being
Fetal distress assoc c:
Congenital issues
Excess bilirubin
Pre-term delivery
Respiratory distress syndrome (RDS)
Lab tests in determining well-being
Alpha-fetoprotein
Used as an indicator for spinabifida, Down’s syndrome, stillbirth, and other chromosome abnormalities.
Bilirubin – spectral curve analysis of amniotic fluid protected from light
Method 1 – Liley scale - plot absorbance at 450 (the delta OD) against estimated gestational age
Method 2 – spectral scan of amniotic fluid looking for a peak at 450 nm
- reflects degree of fetal hemolysis
Must protect specimen from light (usually drawn into a brown tube)
Fetal Fibronectin
Used as an indicator for pre-term delivery
Its presence in weeks 24 – 34 may indicate woman to be at risk for pre-term delivery
More valuable as a negative predictor rather than positive predictor - low levels mean no preterm labor
Surfactant production the basis for other tests
Surfactant consists of
Lecithin
Phosphotidylinosital
Phosphatidylglycerol
Protein
Little, if any sphingomyelin
Fetal Lung Maturity Tests
fetal lungs usually are mature after 37 weeks
L/S ratio
Longest used test for predicting FLM
- lecithin inc c maturity, sphingomyelin stays same
Sensitivity = 96%; Specificity = 68%
Requires 3-4 hours of lab time
Affected by blood and meconium
Technically difficult to perform
Precision at 2.0 (maturity) is fairly low
- Laboratory Procedure:
Chloroform/methanol extraction
Cold acetone precipitation
Thin layer chromatography
Staining/charring of lipids
Measurement of lipid spots
Predicative value = 50/448
11% develop RDS
Phosphatidylglycerol, qualitative
- first detected @ ~37WGA
Sensitivity = 95%; Specificity = 65%
Time varies from 10 minutes to 4 hours
Not affected by blood or meconium
TLC methodology
AmnioStat-FLM
- Amnio-Stat Procedure:
No extraction
0.25 mL amniotic fluid
Immunologic agglutination assay
CLS performs interpretation
Positive when PG > 0.5ug/mL
15 minutes
Negative PG is usually followed up with L/S ratio or TDx FLM
Fluorescence polarization
FLM TDx
AF is filtered to remove debris
Quantitatively measures surfactant/albumin ratio
Run to run precision is 5%
12% will develop RDS
Lamellar body counts - test of choice***
LBC are produced at about 28 weeks
Lamellar bodies represents the structural form of pulmonary surfactant
Attempts to estimate surfactant production in utero
Flow cytometry, light scattering principle
15 minutes to perform
Affected by blood, meconium, mucus
Easy
Precision = 10%
Sensitivity = 100%
Specificity = 59%
LB are counted in the platelet channel of automated cell counter
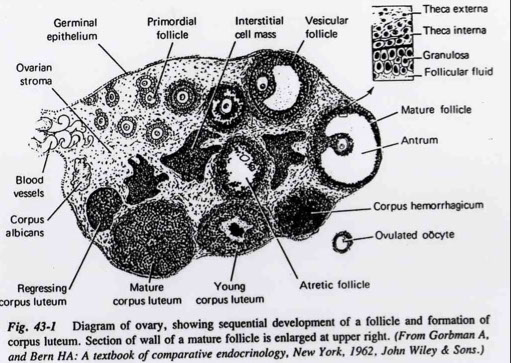
Female Reproductive Anatomy
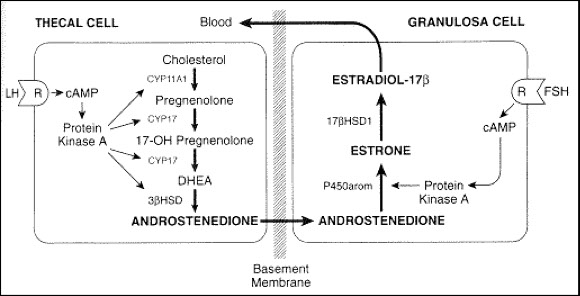
Ovaries
Estradiol – thecal cells
Progesterone – granulosa cells
Fallopian Tubes
Fingerlike projections that guide the ova after expulsion from the ovary
Fertilization occurs here
Uterus
Implantation of the fertilized egg
Lining sloughs off if fertilization does not take place – known as menstruation, period, etc.
Hormone Regulation
Four layers involved
1) Hypothalamus
GnRH
Controlled by dopamine & norepi
2) Anterior pituitary
Release of FSH & LH
Conversion of testosterone to estradiol between the thecal cells and granulosa cells
3) Ovary
LH & FSH causes granulosa cells to produce progesterone from the corpus luteum
Inhibin & progesterone provides negative feedback
4) Uterus
Produces progesterone to support fertilized egg
Females at Puberty
Characterized by 3 major events:
Onset of adrenal androgen secretion
Androstenedione
Dihydroepiandrosterone (DHEA)
Dihydroepiandrosterone sulfate (DHEAS or DHEA SO4)
Decreased sensitivity of the hypothalamus to negative feedback by gonadal steroids leading to increased Gn-RH release
Increased ovarian estradiol secretion and onset of cycles
Estradiol
Most potent
Increases # of glandular cells
Contributes to breast development
Increases osteoblastic activity
Retains Ca & PO4
Enhances secondary sexual characteristics
Used to assess ovarian function
Progesterone
Increases glandular cell secretion
Produced by the placenta during pregnancy
Used to evaluate fertility & the detection of ovulation
Contraception
Inhibition of ovulation by suppressing luteinizing hormone (LH);
Thickening of cervical mucus, thus hampering the transport of sperm;
Possible inhibition of sperm capacitation;
Hampers implantation of the egg
Female Infertility Syndromes
Uterine abnormalities
Tumors
Partial/total destruction of uterus
Asherman’s Syndrome
Tubular abnormalities
Chronic infections
Ovarian abnormalities
Results in anovulation
Abnormal Ovarian Function
Primary ovarian hypofunction
- Genetic disorder – Turner’s Syndrome
45 chromosomes = 44 normal + 1X not 2
Primitive ovaries – unable to ovulate
Increase in LH & FSH
Treatment = HRT does not restore fertility
- Menopause
Median onset = 50
Decreased steroidal feedback at the hypothalamus or pituitary
GnRH, LH, FSH no longer coordinated
Treatment = HRT
http://www.nhlbi.nih.gov/whi/
Secondary ovarian hypofunction
Abnormality lies somewhere along the hypothalmic-pituitary axis
Hypothalmic
GnRH center in hypothalmus is altered
Pituitary hemorrage
Sheehan’s syndrome (post-partum)
Hyperprolactemia
Pituitary tumor secreting prolactin
Anorexia
Delayed hormonal synthesis
Hyperfunction of the Ovaries
Primary – estrogen producing tumors
Decreases in FSH & LH
Secondary – idiopathic
Increases in LH, FSH
Polycystic Ovarian Syndrome (PCOS)
Stein-Levanthal syndrome
Male secondary sexual characteristics
Increased testosterone & LH
Decreased to normal FSH
LH/FSH ratio > 1.5
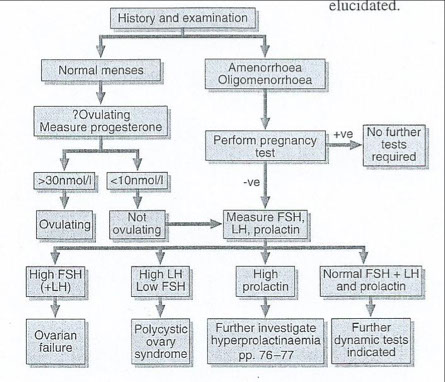
Investigation of female infertility
History & Exam
GnRH test
Clomiphine citrate test
Progesterone withdrawal test
Normal menses vs amenorrhea or oligomenorrhea
The chart on the next slide shows the algorithm for investigating female infertility
Gonadotropin releasing hormone test
Draw baseline LH & FSH
Give patient _______________
Draw blood every 15 for 1 hour
Measure LH & FSH at each draw
Normal response: LH & FSH peaks at 30 to 45 minutes
No Peak: __________________
Clomiphine citrate testing
Draw baseline LH & FSH
Give patient _________for 5 days
Draw blood for next 10 days
Measure LH & FSH at each draw
Normal response: LH should double in concentration from baseline
No Doubling: __________________
Progesterone withdrawl testing
Indirect measure of endogenous estradiol
Used to assess the uterus’ ability to receive a fertilized egg
Give the patient progesterone for 5 days
When the hormone is stopped, uterine bleeding should occur
This indicates the uterus is capable of handling a fetus to term
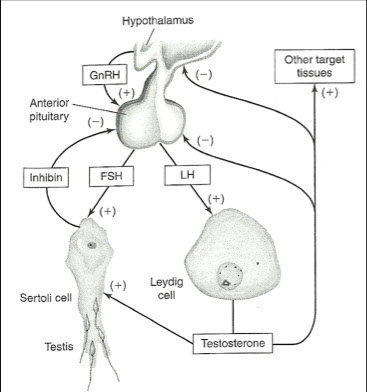
Male anatomy
Leydig cells
Located between and among seminiferous tubules
Site of testosterone synthesis
Sertoli cells
Site of androgen-binding protein
Mediate the first mitotic division of the spermatocytes
Provide necessary environmental conditions for germ cell maturation
Pulsatile release of GnRh
AP releases LH & FSH
LH is major regulator of testosterone production
- Binds to receptors on Leydig cells
- Pulsatile release
- Short half-life
FSH binds to receptors on the Sertoli cells
More constant release
Longer half-life
Negative feedback provided by
LH, testosterone, Inhibin B
LH → hypothalamus & AP
Testosterone → FSH & LH
Inhibin B → FSH
Spermatogenesis
Vital role is played by
Pituitary gonadotropins
FSH acts on the Sertoli cells
LH increases testosterone synthesis in Leydig cells
Testosterone
Stimulates spermatogenesis
Contributes to secretion of semen & ejaculation
Matures the secondary sex characteristics
Feeds back to the hypothalamus to inhibit the release of LH & FSH
Products of metabolism
Androsterone
Dihydrotestosterone
Androstenediol
Etiocholanolone
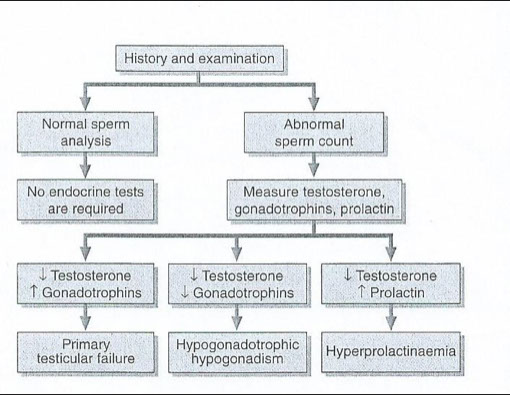
Male infertility
Pretesticular
Due to lesions in the pituitary or hypothalamus
Decreases in LH & FSH lead to decrease in testosterone
Testicular
Congenital
Cryptorchidism
Klinefelter’s syndrome
Acquired
Both result in decreased testosterone & increases in LH & FSH
Post-testicular
Due to functional or mechanical impairment of sperm transport system
No hormone levels involved


Male infertility algorithm